Acid sulfate soil is the common name for soils that contain metal sulfides. In an undisturbed and waterlogged state, these soils may pose no or low risk. However, when disturbed or exposed to oxygen, acid sulfate soils undergo a chemical reaction known as oxidation. Oxidation produces sulfuric acid which has led to these soils being called acid sulfate soils.
How and where acid sulfate soils are formed
Acid sulfate soils are formed by bacterial activity in waterlogged conditions when there is no or little available oxygen.
Naturally occurring bacteria convert sulfate (dissolved salt) from seawater, groundwater or surface water into sulfide (another type of compound that contains sulfur). This sulfide reacts with metals especially iron in the soil sediments or water column, to produce metal sulfides (the main components of acid sulfate soils). To convert the sulfate into sulfide, the bacteria also need a source of energy provided by organic material such as decaying vegetation.
Formation and accumulation of ASS in an inundated scenario (not to scale)

Diagram Figure 2.1 from National Guidance for the Management of Acid Sulfate Soils in Inland Aquatic Ecosystems — Environment Protection and Heritage Council and Natural Resource Management Ministerial Council (2011)
Acid sulfate soils occur in inland and coastal environments.
Inland
While inland acid sulfate soils form naturally in aquatic ecosystems, human activity may also increase their formation and accumulation.
Sulfate is one of the 'raw ingredients' required for the formation of acid sulfate soil, and salts already present in the landscape are a good source of sulfate.
The salts are transported by surface and groundwater flows, and this movement has been greatly altered by human-induced changes to land use and hydrology.
In the right conditions (under water, without oxygen and with bacterial activity) acid sulfate soils are produced when sulfate mixes with metals and organic matter. Aquatic ecosystems with highly regulated water flow are more likely to have acid sulfate soil issues than unregulated aquatic ecosystems. This is because regulation structures such as dams, weirs, locks and barrages help control the amount and timing of water delivery, preventing flushing and allowing acid sulfate soils to build up in submerged soil sediments.
1.1 Formation and accumulation of ASS in an inundated scenario (not to scale)

1.2 Exposure and oxidisation of ASS in a drying scenario (not to scale)

1.3 Rewetting of oxidised ASS in a scenario of higher water availability (not to scale)

Conceptual model of an inland aquatic ecosystem (landscape scale) with ASS in a consecutive sequence. Diagram Figure 1 from National Guidance for the Management of Acid Sulfate Soils in Inland Aquatic Ecosystems — Environment Protection and Heritage Council and Natural Resource Management Ministerial Council (2011)
Coastal
Supratidal iron-staining, Cairns QLD

Photo: B. Powell
Acid sulfate soils occur naturally around Australia's coastline. These soils were formed and deposited in areas that are, or once were, coastal environments.
Coastal acid sulfate soils are commonly found in mangrove forests, saltmarsh, floodplains, and salt- and freshwater wetlands. It is generally assumed that coastal areas with approximate surface elevations less than five metres above mean sea level, Australian Height Datum (AHD) potentially have the necessary requirements for the formation of acid sulfate soils. However, it is important to note that this elevation criterion is only a guide and there are exceptions.
Mining
View of open cut mine from Mt Charlotte, WA

Photo: P. Mitchell
Metal sulfides can also occur in rock and may be associated with coal and shale oil deposits. These metal sulfides formed millions of years ago where seawater, inland lakes or wetlands were once found and they may occur a significant distance inland.
Metal ores are also commonly rich in sulfides. These metal sulfides may be exposed to oxidation during mining, quarrying or excavation operations leading to acid rock drainage which is the generation of sulfuric acid and dissolved metals in water in and around a mine site.
See also:
- Managing Acid and Metalliferous Drainage Handbook
- Global Acid Rock Drainage Guide
- National Guidance for the Management of Acid Sulfate Soils in Inland Aquatic Ecosystems
Where acid sulfate soils are found
Acid sulfate soils occur both inland and along coastlines throughout Australia.
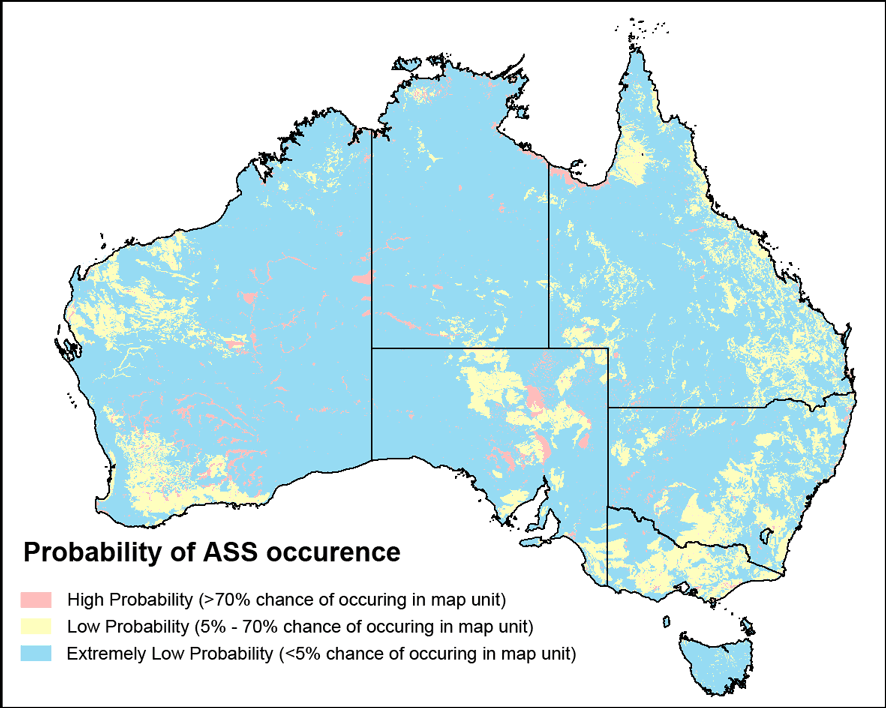
Probability of ASS occurrence in Australia

Source: S. Marvanek, CSIRO
Data and information on the occurrence and likely occurrence of acid sulfate soils is also available through the Atlas of Australian Acid Sulfate Soils. This is a hazard assessment tool which provides information about the broad distribution and properties of acid sulfate soils.
The Atlas is hosted on the Australian Soil Resource Information System and further information about the tool is available from CSIRO.
Why acid sulfate soils are an issue
Jarosite is a field indicator that iron sulfides in acid sulfate soils are oxidising and forming sulfuric acid

Photo: B. Powell
When acid sulfate soils are disturbed or exposed and react with oxygen, they produce sulfuric acid which may be accompanied by certain hazards. Metals may be released from sediments and become bioavailable in the environment, oxygen may be removed from the water column and gases such as hydrogen sulfide, sulfur dioxide and methane may be released.
What makes acid sulfate soils an issue?
Acid sulfate soils in inland aquatic ecosystems may be exposed in situations of limited water availability. This may be because of changes to land use, hydrological regimes, high demand for water, drought and a changing environment, or a combination of these factors.
The majority of Australia's population is concentrated along the coastline and there may be significant development pressure on this land. Urban development and other land use changes such as marinas, canal estates, underground pipes and swimming pools, may result in the disturbance or exposure of acid sulfate soils.
Industrial activities can also disturb or expose acid sulfate soils. Mining, quarrying or excavation operations may expose metal sulfides (such as pyrite) to oxidation, resulting in acidified receiving waters and soils.
Dredging operations may disturb or expose metal sulfides with serious effects if dredging and dredge spoil disposal is not suitably managed.
Unsustainable groundwater abstraction and poorly managed dewatering activities may result in acid sulfate soils being exposed to oxygen when the water table is lowered. This may potentially result in groundwater acidification and release of metals in acid sulfate soil areas and receiving waters.
Agricultural drainage to lower water tables and manage water logging and salinity is also known to cause acidification in susceptible areas.
What may be affected by acid sulfate soils
Environmental values
Environmental values (also known as beneficial uses) are defined under the National Water Quality Management Strategy as particular values or uses of the environment that are important for a healthy ecosystem or for public benefit, welfare, safety or health and which require protection from the effects of pollution, waste discharges and deposits.
Acid sulfate soils may affect the following key environmental values or uses:
- aquatic ecosystems
- primary industries
- recreation and aesthetics
- drinking water
- industrial water, and
- cultural and spiritual values
| Environmental values | Affect |
|---|---|
Aquatic ecosystems | Aquatic ecosystems may be affected by changes to water and soil quality. This can lead to negative effects on the species and ecological communities that depend on this ecosystem. |
Primary industries | Irrigation water may be acidic and/or have high concentrations of metals, which may affect stock drinking water, infrastructure and machinery, and crop growth and yield. Commercial fisheries may be affected by poor water quality that may cause fish kills or disease, and affect human consumption of aquatic foods. |
Recreation and aesthetics | An environment may not be able to be used or enjoyed to the same extent for recreational purposes due to factors including acidic water, odours, loss of aesthetic appeal, loss of fishing amenity and acid-tolerant mosquitoes increasing in number. |
Drinking water | Water quality may be unsafe for human consumption due to factors such as pH change, changes to the concentration of dissolved metals or load of suspended metals and tastes and odours. |
Industrial water | Water may not be suitable for certain industrial purposes. For example, acidified water may corrode metals in the manufacturing process. |
Cultural and spiritual values | Areas of cultural and spiritual significance may be degraded or may not be able to be used for cultural, recreational or consumptive uses. Significant fish and plants may be affected by acidic water, metal contamination or oxygen depletion in water. |
More information
For guidance on protecting environmental values see:
Matters of national environmental significance
The Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) is the Australian Government's central piece of environmental legislation. Under this Act, if a proposed action is likely to have a significant impact on any listed matters of national environmental significance, the action must be referred to the Minister for the Environment. The Minister is then required to make a decision on whether the action requires further assessment and approval under the EPBC Act.
While acid sulfate soils are not explicitly addressed in the EPBC Act, actions which may expose or disturb such soil must be referred to the Minister if they are likely to have a significant impact on any matters of national environmental significance. Please also consult the relevant agency in your state or territory to find out which other legislation and policies may apply.
The nine matters of national environmental significance protected under the EPBC Act are:
- world heritage properties
- national heritage places
- wetlands of international importance (listed under the Ramsar Convention)
- listed threatened species and ecological communities
- migratory species protected under international agreements
- Commonwealth marine areas
- the Great Barrier Reef Marine Park, and
- nuclear actions (including uranium mines)
- a water resource, in relation to coal seam gas development and large coal mining development.
Human health and wellbeing
Human health and wellbeing may be affected by acid sulfate soils in a number of ways. Water quality may be compromised to the extent that it becomes unsafe to drink and food may be contaminated.
Construction sites and drying wetlands may be associated with acidic dust that can expose nearby residents to the risk of eye, throat and skin irritation.
Acidic conditions may lead to increasing numbers of acid-tolerant mosquitoes which can carry diseases like Dengue fever and Ross River virus.
Areas that are valued for recreation and aesthetics may be degraded as the poor water quality may present a public health risk and foul odours may be released from exposed soil.
Cultural and spiritual values may also be degraded, affecting traditional practices and ceremonies, and having negative impacts on physical and mental health and wellbeing.
Iron from acid sulfate soils is known to stimulate harmful algal blooms such as Lyngbya majuscule or Fireweed.
Bernes wetland in the Maria River floodplain, Hastings River catchment, NSW, 2002

Photo: M. Tulau
Plume of dissolved iron in Darawank Creek, Great Lakes Council, NSW, circa 1998

Photo: G. Tuckerman
Built environments
Concrete cancer in pipes Fernbank Creek, Hastings River floodplain, NSW 2001

Photo: G. Atkinson
The effects of acid sulfate soils on built environments may be more obvious.
The ground may subside and lead to structural damage to roads, bridges and buildings.
Acid sulfate soils may cause metal to corrode and concrete, bricks and mortar to break down or crack. Buried metal pipes, fittings and joins may corrode at an accelerated rate.
These effects may lead to structures and materials requiring increased maintenance costs and eventual replacement decades earlier than under normal circumstances.
Economy
The economic costs of disturbing or exposing acid sulfate soils may be considerable.
Expenses are associated with treatment and rehabilitation, impacts on natural and built environment assets, replacement of infrastructure, loss of products, effects on industries such as tourism, agriculture, and fisheries, reduction in property and land values and human health impacts.
Where to get assistance
The effects of acid sulfate soils on the environment can be avoided or minimised with appropriate assessment and management.
Contact the relevant agency in your state or territory for advice specific to your area. See Resources on acid sulfate soils for further details.
Source: http://www.agriculture.gov.au


















Bình luận bài viết